Aurelia
Diversity
There are six species of moon jellyfish in the genus Aurelia. According to the Catalogue of Life’s 2017 Annual checklist, these species are A. aurita, A. colpata, A. labiata, A. limbata, A. maldivensis, and A. solida (Orrell et al., 2017). Aurelia aurita is the type species, or the representative species, of the genus. Although the Catalogue of Life recognizes only six species, recent analysis of polyps, ephyrae, and medusae focused on two additional species that differ morphologically from A. aurita. These species are Aurelia coerulea von Lendenfeld and Aurelia relicta. The study that made these findings also recognizes A. solida as being divergent from A. aurita and further credits geographical isolation for the distinctiveness of these three species, with A. coerulea preferring small bodies of coastal water, A. relicta marine lakes, and A. solida coastal waters with access to the ocean (Scorrano et al., 2017). The differentiation of Aurelia populations that have been separated geographically has some researchers pondering if there are more than just the nine described species. ("Monterey Bay Aquarium", 2018; Orrell, et al., 2017; Scorrano, et al., 2017)
Geographic Range
Aurelia species are found all over the seas of the Northern Hemisphere, including the Atlantic, Pacific, and Indian Oceans (Wildscreen Arkive, 2018). They are also found in smaller bodies of water like the Tokyo Bay, Adriatic Sea, Red Sea, and marine lagoons in temperate regions of Europe (Scorrano et al., 2017). In the U.S., they are found along the coast of California and in the Gulf of Mexico (Monterey, 2018). ("Monterey Bay Aquarium", 2018; "Wildscreen Arkive", 2018; Scorrano, et al., 2017)
- Biogeographic Regions
- nearctic
- palearctic
- indian ocean
- atlantic ocean
- pacific ocean
- mediterranean sea
- Other Geographic Terms
- holarctic
- cosmopolitan
Habitat
Having such a broad geographic distribution means moon jellyfish also have a wide variety of preferred habitats. While A. aurita can survive in environments with a wide range of temperature and salinity values, species like A. limbata need cooler, boreal waters (Scorrano et al., 2017). Preferred temperature ranges from 2-26 degrees Celsius (35.6-78.8 degrees Fahrenheit). Studies have shown that as temperature increases, asexual reproduction in Aurelia increases too, sometimes causing nuisance jellyfish blooms in warmer waters (Pascual et al., 2015). The life cycle of Aurelia is regulated by temperature and season. Ephyrae develop into medusae in the spring but do not reaching full maturity until after the rainy season ends in September (Lo & Chen, 2008). Many jellyfish prefer habitats that possess man-made structures, such as piers, which are ideal for polyps. The proximity to shore has the added benefit of protecting them from larger, predatory jellyfish found in deeper waters (Makabe et al., 2015). (Lo and Chen, 2008; Makabe, et al., 2015; Pascual, et al., 2015; Scorrano, et al., 2017)
- Habitat Regions
- temperate
- tropical
- saltwater or marine
- Other Habitat Features
- estuarine
- intertidal or littoral
Systematic and Taxonomic History
The species Aurelia aurita was previously known as Aurelia flavidula, but this name is no longer recognized (World Register of Marine Species, 2018). Also, the name Aurelia colpata has nomen dubium status (WoRMS, 2018). Although most species of Aurelia have undergone no name changes, nor has the genus name changed, it is worth noting that there is some debate over the number of moon jelly species. Referring back to the “Diversity” section of this account and the research done by Scorrano et al., it is possible that more species of Aurelia are waiting to be named and described. (Cornelius, et al., 2018; Scorrano, et al., 2017)
Members of Aurelia are most closely related to members of the order Rhizostomeae (Hamner & Dawson, 2008). Some synapomorphies of the order Semaeostomeae to which Aurelia belongs include the presence of podocysts (encysted clumps of epidermal cells that allow jellyfish to essentially go dormant in harsh conditions), complex radial canals, and partial circular canals (Dawson & Hamner, 2009). (Dawson and Hamner, 2009; Hamner and Dawson, 2008)
-
- Synapomorphies
-
- Podocysts
- Complex Radial Canals
- Partial Circular Canals
Physical Description
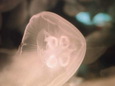
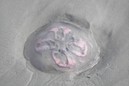
When people picture a jellyfish, they picture Aurelia (Monterey Bay Aquarium, 2018). Adult medusas appear as mostly translucent, saucer shaped domes floating through the water (Wildscreen Arkive, 2018). The diameter of their dome depends on the species with some species growing to 250 mm wide. The only part of a jellyfish that is colored are the four gonads, or reproductive organs, found on the folds of their gastrodermis, the lining of their gastrovascular cavity. These appear as a milky white, light pink, or a light rose colored circle near the center of the jellyfish’s dome. Around their dome are small, thin tentacles similar to cilia found on bacterium but easily visible to the unaided eye. These tentacles move food into pouches, located at different places depending on the species of Aurelia. Moon jellies generally have four oral arms, which appear as longer tentacles that transfer food from the pouches to the mouth (National Oceanic and Atmospheric Administration, 1976). ("Marine Flora and Fauna of the Northeastern United States. Cnidaria: Scyphozoa", 1976; "Monterey Bay Aquarium", 2018; "Wildscreen Arkive", 2018)
While the medusa is the most recognizable form of a jellyfish, there are two other stages to their life cycle (Boero, 2013). The first stage is the polyp, which looks like a small anemone on top of a thin shaft. Polyps can grow to a height of approximately 16 mm (Scorrano et al., 2017). At the top of the polyp stock is a mouth surrounded by tentacles. Depending on the species, Aurelia polyps can have as many as 22 tentacles. The next stage is ephyrae, which are essentially small, flat medusa that can have a diameter up to 4 mm and range in color from brown to orange to milky white. They are characterized by folds around their bodies, called lappets. Like adults, they have a mouth at the center of their bodies (NOAA, 1976). ("Marine Flora and Fauna of the Northeastern United States. Cnidaria: Scyphozoa", 1976; Boero, 2013; Scorrano, et al., 2017)
- Other Physical Features
- ectothermic
- radial symmetry
Reproduction
Aurelia undergo both a sexual and asexual phase of reproduction (Monterey Bay Aquarium, 2018). During the sexual portion of reproduction, males release sperm into the surrounding water, which a nearby female ingests through the mouth. After eggs have developed within pockets inside the mother, they settle as larvae on the ocean floor or another anchoring place (such as a pier). The larvae then develop into polyps, which cycle through feeding and asexual reproductive stages for up to 25 years. During the reproductive stage, polyps undergo strobilation, which releases clones called ephyrae that develop into adult medusa within a few months to repeat the process (Wildscreen Arkive, 2018). ("Monterey Bay Aquarium", 2018; "Wildscreen Arkive", 2018)
- Mating System
- polygynandrous (promiscuous)
Fertilized Aurelia eggs are typically released from the mother as larvae in the fall months (Wildscreen Arkive, 2018). Larvae develop into polyps and begin releasing ephyrae in late fall, early winter. Ephyrae need only a few months to develop into medusa as long as food is plentiful (Lo & Chen, 2008). ("Wildscreen Arkive", 2018; Lo and Chen, 2008)
- Key Reproductive Features
- iteroparous
- gonochoric/gonochoristic/dioecious (sexes separate)
- sexual
- asexual
- fertilization
- ovoviviparous
Once the father jellyfish has released his sperm, his commitment to reproduction is over (Wildscreen Arkive, 2018). The mother’s contribution is also short lived—lasting just long enough for fertilization to occur and for larvae to be released (Lo & Chen, 2008). ("Wildscreen Arkive", 2018; Lo and Chen, 2008)
- Parental Investment
- no parental involvement
Lifespan/Longevity
It is challenging to quantify the life span of jellyfish, as they survive in stages. From strobilation, they enter the ephyrae stage, which lasts six months. Medusas, the adult stage, typically survive an additional six months (Boero, 2013). This is short in comparison to jellyfish polyps that can survive for 25 years. Factors other than natural causes that contribute to the demise of jellyfish include predation by fish, larger jellyfish, and largely variable temperatures. (Boero, 2013)
Behavior
The most social event jellyfish participate in are blooms, or large gatherings. However, these blooms are merely a result of abundant food and ideal weather conditions, which cause bursts of reproduction, rather than purposeful socialization (Boero, 2013). Blooms also occur in areas of overfishing, as the jellyfish do not have to out-compete fish for food (Purcell, 2005). Other than this, there does not appear to be any purposeful interactions between jellies. Medusas and ephyrae move through the water by contracting their domes. The polyp stage of Aurelia is sessile, anchoring itself on the sea floor, rocks, or manmade underwater structures. Jellyfish are notorious for their stingers, which they use in self-defense or for the purpose of killing prey. The cells capable of stinging are called cnidocysts, which inject venomous barbs when in contact with their victim (Boero, 2013). (Boero, 2013; Purcell, 2005)
Communication and Perception
There is little information known about jellyfish communication with each other. It appears that interactions that lead to reproduction or blooms are due to the right environmental conditions, food abundance, and timing. Studies indicate that Aurelia interactions with the environment are largely determined by current. Although little is known about this phenomenon, jellyfish may have the ability to sense that they are in deeper waters and need to return to shallower bodies to prevent being preyed on by larger jellyfish (Makabe et al., 2015). (Makabe, et al., 2015)
- Communication Channels
- tactile
- Perception Channels
- tactile
Food Habits
Zooplankton, including copepods and their larvae, are a staple of Aurelia diets. Medusas have also been known to consume bivalve larvae and fish eggs (Lo & Chen, 2008). As mentioned in a previous section, medusas have pouches around their body for food storage and four oral arms that retrieve the food from storage and deliver it to the mouth (NOAA, 1976). Medusas also have nematocysts, which are cells containing venomous barbs that are injected into prey (Boero, 2013). Polyps of Aurelia thrive on planktonic ciliates, such as Favella taraikaensis, or even small shrimp like those belonging to the genus Artemia (Kamiyama, 2013). ("Marine Flora and Fauna of the Northeastern United States. Cnidaria: Scyphozoa", 1976; Boero, 2013; Kamiyama, 2013; Lo and Chen, 2008)
- Primary Diet
-
carnivore
- piscivore
- eats eggs
- eats non-insect arthropods
- planktivore
Predation
The medusae and ephyrae of Aurelia are preyed on by larger jellyfish, such as those in the genus Chrysaora, Phacellophora, Aequorea, or Drymonema larsoni (Makabe et al., 2015; Encyclopedia of Life; Bayha et al., 2012). They are also at risk of predation by the sunfish Mola mola and leatherback sea turtles Dermochelys coriacea (Bayha et al., 2012). Polyps of Aurelia face their own gastropod and crustacean predators, listed below (Takao et al., 2014). To defend themselves from attack, Aurelia use their stinging cells, cnidocysts, when they come into direct contact with a predator. Research also seems to indicate that Aurelia avoid deeper waters that are home to larger, predatory jellyfish (Makabe et al., 2015). ("Aurelia aurita", 2013; Bayha, et al., 2012; Makabe, et al., 2015; Takao, et al., 2014)
-
- Known Predators
-
- Chrysaora
- Phacellophora
- Aequorea
- Drymonema larsoni
- Mola mola
- Dermochelys coriacea
- Calliostoma unicum
- Pleurobranchaea japonica
- Hermissenda crassicornis
- Sakuraeolis enosimensis
- Sakuraeolis sakuracea
- Rhynchocinetes uritai
- Latreutes anoplonyx
- Hyastenus diacanthus
Ecosystem Roles
In general, moon jellies do not appear to have much interaction with the environment outside of their prey and their predators. Not only do Aurelia prey on fish eggs and larvae, they also compete with them for zooplankton, thus impacting fish populations (Lo & Chen, 2008). In large quantities (blooms), they have been known to cause problems. For example, an Aurelia bloom may over consume the zooplankton in the area, indirectly causing a phytoplankton bloom without zooplankton to keep them in check (Lo & Chen, 2008). (Lo and Chen, 2008)
- Ecosystem Impact
- keystone species
Economic Importance for Humans: Positive
Moon jellies are the subject of thousands of studies about their tendencies and ability to survive as the environment changes as well as how they impact other organisms. Aside from their appeal as a research specimen, jellyfish draw a lot of attention in aquariums and other tourist hot spots. Jellyfish watching has become a major attraction in Palau and may be catching on in other coastal places (Boero, 2013). (Boero, 2013)
- Positive Impacts
- ecotourism
Economic Importance for Humans: Negative
Because Aurelia compete with fish populations, they have periodically decreased fishery populations for human consumption. Another indirect way in which jellyfish impact humans is their tendency to block intakes for power plant cooling water (Lo & Chen, 2008). As far as direct impact goes, jellyfish have a reputation for stinging tourists, sometimes causing significant injuries that require time in the hospital (Pascual et al., 2015). (Lo and Chen, 2008; Pascual, et al., 2015)
- Negative Impacts
-
injures humans
- bites or stings
- venomous
Conservation Status
Moon jellyfish are of conservation concern (Monterey Bay Aquarium, 2018). They are found in abundance around the globe. ("Monterey Bay Aquarium", 2018)
-
- IUCN Red List [Link]
- Not Evaluated
Other Comments
The prevalence of research on blooms, where they occur, and why they occur is increasing in attempts to forecast future blooms, allowing the tourism and fishery industries in the impacted communities to prepare. Some of the methods researchers use to monitor jelly populations include counting jellyfish from boats, planes, submersibles, and even radio tracking (Boero, 2013). Researchers couple this information with wind speed/direction, current, and temperature to aid in observing the migration of jellyfish populations (Boero, 2013). Because of the impact blooms have on the ecosystem, they have become a focal point for citizen science. Researchers use reports of jellyfish washing up on beaches or gathering off shore to estimate the number of jellies and pictures taken by people to identify the species of jellyfish that are amassing. Boero acknowledges that it is difficult to actually forecast where blooms will move next, but with the help of citizen science, it is easier to monitor where jellyfish are in real time (2013). (Boero, 2013)
Contributors
McKenzie Fletcher (author), Colorado State University, Tanya Dewey (editor), University of Michigan-Ann Arbor.
Glossary
- Atlantic Ocean
-
the body of water between Africa, Europe, the southern ocean (above 60 degrees south latitude), and the western hemisphere. It is the second largest ocean in the world after the Pacific Ocean.

- Nearctic
-
living in the Nearctic biogeographic province, the northern part of the New World. This includes Greenland, the Canadian Arctic islands, and all of the North American as far south as the highlands of central Mexico.

- Pacific Ocean
-
body of water between the southern ocean (above 60 degrees south latitude), Australia, Asia, and the western hemisphere. This is the world's largest ocean, covering about 28% of the world's surface.

- Palearctic
-
living in the northern part of the Old World. In otherwords, Europe and Asia and northern Africa.

- asexual
-
reproduction that is not sexual; that is, reproduction that does not include recombining the genotypes of two parents
- carnivore
-
an animal that mainly eats meat
- coastal
-
the nearshore aquatic habitats near a coast, or shoreline.
- cosmopolitan
-
having a worldwide distribution. Found on all continents (except maybe Antarctica) and in all biogeographic provinces; or in all the major oceans (Atlantic, Indian, and Pacific.
- ecotourism
-
humans benefit economically by promoting tourism that focuses on the appreciation of natural areas or animals. Ecotourism implies that there are existing programs that profit from the appreciation of natural areas or animals.
- ectothermic
-
animals which must use heat acquired from the environment and behavioral adaptations to regulate body temperature
- estuarine
-
an area where a freshwater river meets the ocean and tidal influences result in fluctuations in salinity.
- fertilization
-
union of egg and spermatozoan
- holarctic
-
a distribution that more or less circles the Arctic, so occurring in both the Nearctic and Palearctic biogeographic regions.

Found in northern North America and northern Europe or Asia.
- internal fertilization
-
fertilization takes place within the female's body
- intertidal or littoral
-
the area of shoreline influenced mainly by the tides, between the highest and lowest reaches of the tide. An aquatic habitat.
- iteroparous
-
offspring are produced in more than one group (litters, clutches, etc.) and across multiple seasons (or other periods hospitable to reproduction). Iteroparous animals must, by definition, survive over multiple seasons (or periodic condition changes).
- keystone species
-
a species whose presence or absence strongly affects populations of other species in that area such that the extirpation of the keystone species in an area will result in the ultimate extirpation of many more species in that area (Example: sea otter).
- motile
-
having the capacity to move from one place to another.
- native range
-
the area in which the animal is naturally found, the region in which it is endemic.
- ovoviviparous
-
reproduction in which eggs develop within the maternal body without additional nourishment from the parent and hatch within the parent or immediately after laying.
- piscivore
-
an animal that mainly eats fish
- planktivore
-
an animal that mainly eats plankton
- polygynandrous
-
the kind of polygamy in which a female pairs with several males, each of which also pairs with several different females.
- radial symmetry
-
a form of body symmetry in which the parts of an animal are arranged concentrically around a central oral/aboral axis and more than one imaginary plane through this axis results in halves that are mirror-images of each other. Examples are cnidarians (Phylum Cnidaria, jellyfish, anemones, and corals).
- reef
-
structure produced by the calcium carbonate skeletons of coral polyps (Class Anthozoa). Coral reefs are found in warm, shallow oceans with low nutrient availability. They form the basis for rich communities of other invertebrates, plants, fish, and protists. The polyps live only on the reef surface. Because they depend on symbiotic photosynthetic algae, zooxanthellae, they cannot live where light does not penetrate.
- saltwater or marine
-
mainly lives in oceans, seas, or other bodies of salt water.
- sessile
-
non-motile; permanently attached at the base.
Attached to substratum and moving little or not at all. Synapomorphy of the Anthozoa
- sexual
-
reproduction that includes combining the genetic contribution of two individuals, a male and a female
- tactile
-
uses touch to communicate
- temperate
-
that region of the Earth between 23.5 degrees North and 60 degrees North (between the Tropic of Cancer and the Arctic Circle) and between 23.5 degrees South and 60 degrees South (between the Tropic of Capricorn and the Antarctic Circle).
- tropical
-
the region of the earth that surrounds the equator, from 23.5 degrees north to 23.5 degrees south.
- venomous
-
an animal which has an organ capable of injecting a poisonous substance into a wound (for example, scorpions, jellyfish, and rattlesnakes).
References
2013. Aurelia aurita. Online: Encyclopedia of Life. Accessed March 04, 2018 at http://eol.org/pages/203484/details.
2018. "Aurelia aurita" (On-line). National Center for Biotechnology Information. Accessed January 25, 2018 at https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=6145.
National Oceanic and Atmospheric Administration. Marine Flora and Fauna of the Northeastern United States. Cnidaria: Scyphozoa. Washington D.C.: U.S. Department of Commerce. 1976. Accessed January 25, 2018 at http://www.vims.edu/library/GreyLit/OA/MFFNEUS/Cnidaria%20Scyphozoa.pdf.
2018. "Monterey Bay Aquarium" (On-line). Moon Jelly. Accessed January 25, 2018 at https://www.montereybayaquarium.org/animal-guide/invertebrates/moon-jelly.
2018. "Wildscreen Arkive" (On-line). Common jellyfish (Aurelia aurita). Accessed January 25, 2018 at http://www.arkive.org/common-jellyfish/aurelia-aurita/image-A23322.html.
Bayha, K., W. Graham, J. Higgins III, H. Fletcher. 2012. Predation potential of the jellyfish Drymonema larsoni Bayha & Dawson (Scyphozoa: Drymonematidae) on the moon jellyfish Aurelia sp. in the northern Gulf of Mexico, 690(1): 189-197.
Boero, F. 2013. Review of Jellyfish Blooms in the Mediterranean and Black Sea. General Fisheries Commission for the Mediterranean.Studies and Reviews, 92: 1-53.
Cornelius, P., A. Collins, G. Jarms, A. Morandini. 2018. "World Register of Marine Species" (On-line). Accessed January 25, 2018 at http://www.marinespecies.org/aphia.php?p=taxdetails&id=135306.
Dawson, M., W. Hamner. 2009. A character-based analysis of the evolution of jellyfish blooms: adaptation and exaptation. Hydrobiologia, 616: 193-215.
Hamner, W., M. Dawson. 2008. A review and synthesis on the systematics and evolution of jellyfish blooms: advantageous aggregations and adaptive assemblages. Hydrobiologia, 616: 161-191.
Kamiyama, T. 2013. Planktonic Ciliates as Food for the Scyphozoan Aurelia Aurita [S.L.]: Effects on Asexual Reproduction of the Polyp Stage. Journal of Experimental Marine Biology and Ecology, 445: 21-28.
Lo, W., I. Chen. 2008. Population Succession and Feeding of Scyphomedusae, Aurelia Aurita, in a Eutrophic Tropical Lagoon in Taiwan. Estuarine, Coastal and Shelf Science, 76: 227-238.
Makabe, R., H. Takeoka, S. Uye. 2015. Offshore Dispersion of Ephyrae and Medusae of Aurelia Aurita S.L. [Cnidaria: Scyphozoa] from Port Enclosures: Physical and Biological Factors. Journal of Marine Systems, 152: 75-82.
Orrell, T., N. Bailly, T. Bourgoin, W. Decock, A. De Wever, E. Nieukerken, J. Zarucchi, L. Penev. 2017. "Species 2000 & ITIS Catalogue of Life" (On-line). 2017 Annual Checklist. Accessed January 25, 2018 at http://www.catalogueoflife.org/annual-checklist/2017/details/species/id/d166c9f3ebf56703412c660df26556f0/source/tree.
Pascual, M., V. Fuentes, A. Canepa, D. Atienza, J. Gili, J. Purcell. 2015. Temperature Effects on Asexual Reproduction of the Scyphozoan Aurelia Aurita S.L.: Differences between Exotic [Baltic and Red Seas] and Native [Mediterranean Sea] Populations. Marine Ecology, 36: 994-1002.
Purcell, J. 2005. Climate Effects on Formation of Jellyfish and Ctenophore Blooms: A Review. Marine Biological Association of the United Kingdom, 85: 461-476.
Scorrano, S., G. Aglieri, F. Boero, M. Dawson, S. Piraino. 2017. Unmasking Aurelia species in the Mediterranean Sea: an integrative morphometric and molecular approach. Zoological Journal of the Linnean Society, 180(2): 243-267.
Takao, M., H. Okawachi, S. Uye. 2014. Natural predators of polyps of Aurelia aurita s.l. (Cnidaria: Scyphozoa: Semaeostomeae) and their predation rates. Plankton and Benthos Research, 9: 105-113.