Petromyzon marinusEel sucker(Also: Green lamprey; Lamper; Shad lamprey; Spotted lamprey)
Geographic Range
Sea lampreys are native to the Atlantic Ocean. Between the months of March and October, they can be found along the coast of the United States as far north as Massachusetts and as far south as South Carolina. In October, North American lampreys migrate south along the Atlantic coast to warmer climates, some travelling as far south as Florida. Sea lampreys can also be found along the Atlantic coast of Europe as far north as Norway and ranging as far south as the Mediterranean. In October, they can travel as far south as Africa and to parts of the Indian coast. Sea lampreys have also been introduced to the Great Lakes region of the United States many times over within the past 200 years. Reports of this species in Lake Ontario date back to the early 1800s. Great Lake sea lampreys must first travel through the Gulf of St. Lawrence in order to gain access to the Atlantic coastal region. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000; Jenkins and Burkhead, 1993; Lavis, et al., 2001; Lavis, et al., 2003; Nikitina, et al., 2009; Rohde, et al., 1994; Trautman, 1981)
- Biogeographic Regions
- nearctic
- palearctic
- oriental
- atlantic ocean
- mediterranean sea
Habitat
Sea lampreys are anadromous, and migration is triggered by changes in water temperature. In general, they prefer shallow coastal areas, though they are found at depths between 0.91 and 4.57 m. Young lampreys are hatched in gravel or rock beds in small, freshwater streams and rivers. After the larval stage, they migrate into saltwater ocean habitats. They return to freshwater to lay their eggs. Sea lampreys thrive in systems where the following are present: 1) waterways lacking obstructions (like dams or waterfalls) with clean sand and gravel areas for spawning; 2) sand beds free of pollutants with a large supply of organic matter for their developing young; and 3) large waterways with a plentiful supply of fish to serve as hosts for their fully developed offspring. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000; Jenkins and Burkhead, 1993; Nikitina, et al., 2009; Rohde, et al., 1994; Trautman, 1981)
- Habitat Regions
- saltwater or marine
- freshwater
- Aquatic Biomes
- lakes and ponds
- rivers and streams
- coastal
-
- Range depth
- 0.91 to 4.57 m
- 2.99 to 14.99 ft
Physical Description
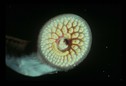
Sea lampreys, Petromyzon marinus, belong to a group of fish called Agnathans, which lack a jaw. Although classified in the subphylum Vertebrata, this species lacks vertebrae, and their entire skeleton is cartilaginous. Commonly known for their smooth, scaleless physique and long cylindrical bodies, they are often misidentified as eels. Sea lampreys also lack swim bladders and a lateral line system. Members of this species have a visible eye spot located on each side of their head behind a single nostril and above a set of seven gill openings. Their mouth takes on an oval shape while attached to their host, but once opened it becomes larger than the head and pharynx together. Inside the oval-shaped mouth are numerous rows of large teeth pointing inward. Sea lampreys have two dorsal fins but lack any paired fins. When spawning occurs, males develop a distinct ridge along their back and females develop a pronounced fold of skin behind their vent. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000; Jenkins and Burkhead, 1993; Rohde, et al., 1994; Trautman, 1981)
Sea lampreys are the largest and most aggressive species of lamprey, ranging from 15.2 to 30 cm in length as juveniles and 30 to 100 cm in length as adults. Adults can weigh up to 2.5 kg. Besides length, there are several key differences between adult and young sea lampreys. Color is often a good indicator of age; larvae generally are dark, greenish brown with a light grey underbelly, while adults are brownish grey and tend to lighten in color when about to spawn. Another key difference involves the dorsal fins; while separate in young lampreys, the dorsal fins migrate closer together as sea lampreys reach adulthood. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000; Jenkins and Burkhead, 1993; Rohde, et al., 1994; Trautman, 1981)
- Other Physical Features
- bilateral symmetry
- polymorphic
- Sexual Dimorphism
- sexes shaped differently
-
- Range mass
- 1 to 2.5 kg
- 2.20 to 5.51 lb
-
- Range length
- 15.2 to 30 cm
- 5.98 to 11.81 in
Development
There are four stages in the life cycle of sea lampreys, which usually spans 18 months but can last as long as 5 years. The first of these stages is the spawning phase, which occurs during spring and early summer. From April to June, sea lampreys search freshwater rivers and streams, seeking an ideal location in which to construct a nest and lay their eggs. Once the area is selected, male sea lampreys construct a nest, often moving rocks to create a large indenture or depression in the river or lake bed. A female then lays 30,000 to 100,000 eggs, which the male externally fertilizes. Both male and female adult sea lampreys float away and die soon after spawning. Unique to this phase is the disintegration of the digestive system; adult sea lampreys cannot feed while spawning. During the second phase, fertilized eggs settle into the sand or gravel and begin to grow. Within a few weeks, the eggs hatch and the larvae burrow further into the sand or gravel. Larvae filter-feed on algae and other aquatic organic matter. This larval phase can last for more than three years. In the third phase, known as transformation, larvae metamorphose into adult sea lampreys. During this phase sea lampreys develop a mouth, teeth and eyes. They also migrate to larger bodies of water, such as oceans or freshwater systems like the Great Lakes. Sea lampreys remain in this habitat for 12 to 18 months as a mature adult and begin to feed, attaching themselves to fish. This is known as the parasitic phase, during which sexual reproductive organs develop. (Bence, et al., 2003; Bryan, et al., 2005; Nikitina, et al., 2009; "Sea Lamprey Management Program", 2010; Bence, et al., 2003; Bryan, et al., 2005; "The Great Lakes Fishery Commission", 2000; Nikitina, et al., 2009)
- Development - Life Cycle
- metamorphosis
- indeterminate growth
Reproduction
Little is known about the mating systems of sea lampreys. It is thought that male sea lampreys emit a pheromone composed of bile acids that alerts ovulating females to their presence. This signal may also be related to mating preferences and may be sent over large distances. Male sea lampreys selectively dig holes into river or stream bottoms and fertilize eggs once the female has laid them. This external fertilization allows multiple males to fertilize eggs. (Bryan, et al., 2005; "The Great Lakes Fishery Commission", 2000; Lavis, et al., 2001; Lavis, et al., 2003; Lesinski, 1996; Li, et al., 2003; Rohde, et al., 1994; Trautman, 1981)
- Mating System
- polygynous
From April to June, female sea lampreys lay between 30,000 and 100,000 eggs. These eggs are fertilized externally by males. Fertilized eggs hatch in 3 to 8 weeks. Larvae spend 1 to 3 years filter-feeding and do not associate with other sea lampreys. By 3 to 5 years of age, sea lampreys reach sexual maturity. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000; McLaughlin, et al., 2007; Somervill, 2008)
- Key Reproductive Features
- seasonal breeding
- sexual
- fertilization
- oviparous
-
- Breeding interval
- Sea lampreys breed once at the end of their lifetime.
-
- Breeding season
- Sea lampreys breed between April and June.
-
- Range number of offspring
- 30,000 to 100,000
-
- Range gestation period
- 3 to 8 weeks
-
- Range age at sexual or reproductive maturity (female)
- 3 to 5 years
-
- Range age at sexual or reproductive maturity (male)
- 3 to 5 years
Male sea lampreys selectively locate a nesting area. Sea lampreys do not allocate energy toward parental investment after laying and fertilizing eggs, as both male and female sea lampreys die shortly after spawning. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000; Somervill, 2008; Trautman, 1981)
- Parental Investment
- no parental involvement
-
pre-fertilization
- provisioning
-
protecting
- male
- female
- pre-hatching/birth
Lifespan/Longevity
Sea lampreys die soon after spawning. They can survive up to 5 years in the wild waiting for the opportune time to reproduce. Most lampreys, however, live 1.5 to 5 years in the wild. ("Sea Lamprey Management Program", 2010; Bryan, et al., 2005; "The Great Lakes Fishery Commission", 2000; McLaughlin, et al., 2007; Somervill, 2008)
-
- Range lifespan
Status: wild - 1.5 to 5 years
- Range lifespan
-
- Typical lifespan
Status: wild - 1.5 to 5 years
- Typical lifespan
Behavior
Sea lampreys are a very motile and live their adult life as parasitic organisms. Specific behavioral patterns of this species, however, are not well studied. Although individuals are not known to interact as larvae, adults are predominantly found in groups or colonies while attached to a host. During spawning, sea lampreys interact using pheromones. Due to morphological and physiological changes necessary to reach the spawning phase, including disintegration of the digestive system, sea lampreys cease feeding when spawning. This species is the largest and most aggressive species of lamprey. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000; Jenkins and Burkhead, 1993; Rohde, et al., 1994; Somervill, 2008; Trautman, 1981)
Home Range
Sea lampreys do not have a home range and do not defend a territory. ("Sea Lamprey Management Program", 2010; Lavis, et al., 2003; Somervill, 2008)
Communication and Perception
Communication patterns of sea lampreys are not well known. It is thought male sea lampreys emit a pheromone composed of bile acids that alerts ovulating females to their presence. This signal may also be related to mating preferences and may be sent over large distances. (Lavis, et al., 2001; Lavis, et al., 2003; Li, et al., 2003; Rohde, et al., 1994; Trautman, 1981)
- Other Communication Modes
- pheromones
Food Habits
Newly hatched larval sea lampreys are freshwater filter-feeders that consume detritus, algae, and other organic material found at river bottoms. Once in a saline environment (or in the Great Lakes), sea lampreys develop parasitic abilities, attach themselves to a fish and ingest their blood and skin. Sea lampreys ultimately breaks down the fish while the fish is still alive. This species is capable of attaching itself to a variety of species of fish and does not seem to have a preference of host species. Once sea lampreys reach sexual maturity, they no longer feed. (Bryan, et al., 2005; Lavis, et al., 2001; Lavis, et al., 2003; Somervill, 2008)
- Primary Diet
-
carnivore
- piscivore
- sanguivore
- eats body fluids
-
herbivore
- algivore
- omnivore
- detritivore
- Animal Foods
- fish
- blood
- body fluids
- carrion
- Plant Foods
- algae
- Other Foods
- detritus
- Foraging Behavior
- filter-feeding
Predation
Sea lampreys do not have many known predators, and their most common predator are humans. While sea lampreys in the Great Lakes region are often killed in preservation efforts of native fish, sea lampreys are also trapped in Europe, Asia, and India to be consumed and are even considered a delicacy. Many European countries capture sea lampreys and use them in a variety of dishes. ("Sea Lamprey Management Program", 2010; Bryan, et al., 2005; "The Great Lakes Fishery Commission", 2000)
-
- Known Predators
-
- humans Homo sapiens
Ecosystem Roles
Parasitic sea lampreys have a detrimental effect on fish within their ecosystem. Specifically, sea lampreys feed on salmon, lake trout, rainbow trout, whitefish, chubs, burbot, walleye, and some catfish. Fish populations as as well as those industries that depend on fish are declining. Sea lampreys have no known predators except humans. In the Great Lakes region, a small percentage of sea lampreys carried cestode parasites or roundworms during some portion of its life. Of these parasites, only roundworms caused severe damage to their lamprey hosts. ("Sea Lamprey Management Program", 2010; Dawson and Jones, 2006; Mandenjian and Desorcie, 2010; Mandenjian, et al., 2003; McLain, 1952; McLaughlin, et al., 2007; Nikitina, et al., 2009)
- Ecosystem Impact
- parasite
- salmon Salmo salar
- lake trout Salvelinus namaycush
- rainbow trout Oncorhynchus mykiss
- whitefish Coregonus clupeaformis
- chubs Gila ditaenia
- burbot Lota iota
- walleye Sander vitreus
- catfish Siluriformes
Economic Importance for Humans: Positive
Sea lampreys are considered a delicacy in many foreign countries like Asia and India and are harvested for food. ("Sea Lamprey Management Program", 2010; "The Great Lakes Fishery Commission", 2000)
- Positive Impacts
- food
Economic Importance for Humans: Negative
Because of their over-aggressive behavior and generalist diet, sea lampreys parasitize many species of fish, contributing to the severe decline of commercial fishing industries, including those on the Atlantic coast of North America and in the Great Lakes region. Each sea lamprey kills more than 18.2 kg of fish each year. This species has parasitized many species of native fish in the Great Lakes region since the early 1800s, leading to the collapse of the Great Lakes commercial fishing industry and costing it millions of dollars. Population declines of native fish in the region have also negatively impacted sport fishing and tourism. In one case, a sea lamprey bit a human, though this is thought to have been accidental. ("Sea Lamprey Management Program", 2010; Baily, 2009; Bryan, et al., 2005; "The Great Lakes Fishery Commission", 2000)
Conservation Status
Sea lampreys are not protected. Indeed, as invasive species, efforts are in place to eradicate them from the Great Lakes region. Both federal and state governments have created programs to manage populations of and educate the community about this invasive, harmful species. Barriers and traps are set in the waterways to capture adult sea lampreys before they reproduce. Lampricides are also added to prime habitat of sea lampreys. These treatments specifically target lampreys and are designed not to harm other species. Each program has been tested for several years and has proven an effective control of sea lampreys. ("Sea Lamprey Management Program", 2010; Baily, 2009; Bence, et al., 2003; Dawson and Jones, 2006; "The Great Lakes Fishery Commission", 2000)
-
- IUCN Red List
-
Least Concern
More information
-
- IUCN Red List
-
Least Concern
More information
-
- US Federal List
- No special status
-
- CITES
- No special status
-
- State of Michigan List
- No special status
Contributors
Selisha Cherry (author), Radford University, Karen Powers (editor), Radford University, Gail McCormick (editor), Animal Diversity Web Staff.
Glossary
- Atlantic Ocean
-
the body of water between Africa, Europe, the southern ocean (above 60 degrees south latitude), and the western hemisphere. It is the second largest ocean in the world after the Pacific Ocean.

- Nearctic
-
living in the Nearctic biogeographic province, the northern part of the New World. This includes Greenland, the Canadian Arctic islands, and all of the North American as far south as the highlands of central Mexico.

- Palearctic
-
living in the northern part of the Old World. In otherwords, Europe and Asia and northern Africa.

- bilateral symmetry
-
having body symmetry such that the animal can be divided in one plane into two mirror-image halves. Animals with bilateral symmetry have dorsal and ventral sides, as well as anterior and posterior ends. Synapomorphy of the Bilateria.
- carnivore
-
an animal that mainly eats meat
- carrion
-
flesh of dead animals.
- chemical
-
uses smells or other chemicals to communicate
- coastal
-
the nearshore aquatic habitats near a coast, or shoreline.
- colonial
-
used loosely to describe any group of organisms living together or in close proximity to each other - for example nesting shorebirds that live in large colonies. More specifically refers to a group of organisms in which members act as specialized subunits (a continuous, modular society) - as in clonal organisms.
- detritivore
-
an animal that mainly eats decomposed plants and/or animals
- detritus
-
particles of organic material from dead and decomposing organisms. Detritus is the result of the activity of decomposers (organisms that decompose organic material).
- external fertilization
-
fertilization takes place outside the female's body
- fertilization
-
union of egg and spermatozoan
- filter-feeding
-
a method of feeding where small food particles are filtered from the surrounding water by various mechanisms. Used mainly by aquatic invertebrates, especially plankton, but also by baleen whales.
- food
-
A substance that provides both nutrients and energy to a living thing.
- freshwater
-
mainly lives in water that is not salty.
- herbivore
-
An animal that eats mainly plants or parts of plants.
- indeterminate growth
-
Animals with indeterminate growth continue to grow throughout their lives.
- introduced
-
referring to animal species that have been transported to and established populations in regions outside of their natural range, usually through human action.
- metamorphosis
-
A large change in the shape or structure of an animal that happens as the animal grows. In insects, "incomplete metamorphosis" is when young animals are similar to adults and change gradually into the adult form, and "complete metamorphosis" is when there is a profound change between larval and adult forms. Butterflies have complete metamorphosis, grasshoppers have incomplete metamorphosis.
- motile
-
having the capacity to move from one place to another.
- native range
-
the area in which the animal is naturally found, the region in which it is endemic.
- omnivore
-
an animal that mainly eats all kinds of things, including plants and animals
- oriental
-
found in the oriental region of the world. In other words, India and southeast Asia.

- oviparous
-
reproduction in which eggs are released by the female; development of offspring occurs outside the mother's body.
- parasite
-
an organism that obtains nutrients from other organisms in a harmful way that doesn't cause immediate death
- pheromones
-
chemicals released into air or water that are detected by and responded to by other animals of the same species
- piscivore
-
an animal that mainly eats fish
- polygynous
-
having more than one female as a mate at one time
- polymorphic
-
"many forms." A species is polymorphic if its individuals can be divided into two or more easily recognized groups, based on structure, color, or other similar characteristics. The term only applies when the distinct groups can be found in the same area; graded or clinal variation throughout the range of a species (e.g. a north-to-south decrease in size) is not polymorphism. Polymorphic characteristics may be inherited because the differences have a genetic basis, or they may be the result of environmental influences. We do not consider sexual differences (i.e. sexual dimorphism), seasonal changes (e.g. change in fur color), or age-related changes to be polymorphic. Polymorphism in a local population can be an adaptation to prevent density-dependent predation, where predators preferentially prey on the most common morph.
- saltwater or marine
-
mainly lives in oceans, seas, or other bodies of salt water.
- sanguivore
-
an animal that mainly eats blood
- seasonal breeding
-
breeding is confined to a particular season
- sexual
-
reproduction that includes combining the genetic contribution of two individuals, a male and a female
- solitary
-
lives alone
- visual
-
uses sight to communicate
References
2010. "Sea Lamprey Management Program" (On-line). U.S. Fish and Wildlife Service. Accessed September 24, 2010 at http://midwest.fws.gov/Marquette/index.html.
Great Lakes Fishery Commission. 2000. "The Great Lakes Fishery Commission" (On-line pdf). Sea lamprey. Accessed September 12, 2010 at http://www.seagrant.umn.edu/downloads/x106.pdf.
Baily, N. 2009. "Petromyzon marinus Linnaeus, 1758." (On-line). World Database of Marine Species. Accessed September 14, 2010 at http://www.marinespecies.org/aphia.php?p=taxdetails&id=101174.
Bence, J., . Bergstedt, G. Christie, P. Cochran, M. Ebener, J. Koonce, M. Rutter, W. Swink. 2003. Sea lamprey (Petromyzon marinus) parasite-host interactions in the Great Lakes. Journal of Great Lakes Research, 29/1: 253-282.
Bryan, M., D. Zalinski, K. Filcek, S. Libants, W. Li, K. Scribner. 2005. Patterns of invasion and colonization of the sea lamprey (Petromyzon marinus) in North America as revealed by. Molecular Ecology, 14/12: 3757–3773.
Dawson, H., M. Jones. 2006. Factors affecting recruitment dynamics of Great Lakes sea lamprey (Petromyzon marinus) populations. Journal of Great Lakes Research, 32/3: 353-360.
Jenkins, R., N. Burkhead. 1993. Freshwater Fishes of Virginia. Bethesda, Maryland: American Fisheries Society.
Lavis, D., A. Hallett, E. Koon, T. McAuley. 2003. History of and advances in barriers as an alternative method to suppress sea lampreys in the Great Lakes. Journal of Great Lakes Research, 29/1: 362-372.
Lavis, D., M. Henson, D. Johnson, E. Koon, D. Ollila. 2001. A case history of sea lamprey control in Lake Michigan: 1979 to 1999. Journal of Great Lakes Research, 29/1: 584-598.
Lesinski, J. 1996. Exotic Invaders: Killer Bees, Fire Ants, and Other Alien Species are Infesting America!. Chicago: Walker & Company.
Li, W., M. Siefkes, A. Scott, J. Teeter. 2003. Sex pheromone communication in the sea lamprey: Implications for integrated management. Journal of Great Lakes Research, 29/1: 85-94.
Mandenjian, C., P. Cochran, R. Bergstedt. 2003. Seasonal patterns in growth, blood consumption, and effects on hosts by parasitic-phase sea lampreys in the Great Lakes: an individual-based model approach. Journal of Great Lakes Research, 29/1: 332-346.
Mandenjian, C., T. Desorcie. 2010. Lake Trout population dynamics in the northern refuge of Lake Michigan: implications for future rehabilitation. North American Journal of Fisheries Management, 30/3: 629-641.
McLain, A. 1952. Diseases and Parasites of the Sea Lamprey, Petromyzon Marinus, in the Lake Huron Basin. Transactions of the American Fisheries Society, 81/1: 94-100.
McLaughlin, R., A. Hallett, T. Pratt, L. O'Connor, D. McDonald. 2007. Research to guide use of barriers, traps, and fishways to control sea lamprey. Journal of Great Lakes Research, 33/2: 7-19.
Nikitina, N., M. Bronner-Fraser, T. Sauka-Spengler. 2009. The sea lamprey Petromyzon marinus: a model for evolutionary and developmental biology. Cold Spring Harb Protocols, 113: 1-39.
Rohde, F., R. Arndt, D. Lindquist, J. Parnell. 1994. Freshwater Fishes of the Carolinas, Virginia, Maryland, and Delaware. Chapel Hill,North Carolina: The University of North Carolina Press.
Somervill, B. 2008. Sea Lamprey. New York: Cherry Lake Publishing.
Trautman, M. 1981. The Fishes Of Ohio. Ohio: Ohio State University Press.